Research Overview
The Division of Bioorganic Chemistry conducts research aimed at contributing to medicinal chemistry and life sciences by designing and creating molecules with unique and useful functions. Based on organic chemistry, we develop functional molecules incorporated with our original ideas, which will lead to the elucidation of new life phenomena and the elucidation of the pathogenic mechanisms of diseases and their treatment.
1 Novel approach to control the activity and physical properties of medium-sized molecules.
References:
• H. Kitagawa, M. Kikuchi, S. Sato, H. Watanabe, N. Umezawa, M. Kato, Y. Hisamatsu, T. Umehara, T. Higuchi, "Structure-based identification of potent lysine-specific demethylase 1 inhibitor peptides and temporary cyclization to enhance proteolytic stability and cell growth-inhibitory activity." J. Med. Chem., 64, 3707-3719, 2021.
• Y. Amano, N. Umezawa, S. Sato, H. Watanabe, T. Umehara, T. Higuchi, "Activation of lysine-specific demethylase 1 inhibitor peptide by redox-controlled cleavage of a traceless linker."
Bioorg. Med. Chem., 25, 1227-1234, 2017.
• N. Umezawa, Y. Noro, K. Ukai, N. Kato, T. Higuchi, "Photocontrol of peptide function: Backbone cyclization strategy with photocleavable amino acid." ChemBioChem, 12, 1694-1698, 2011.
Our proposed approach to controlling activity and physical properties using temporary cyclization.
2 Development of functional peptide derivatives based on conformational stabilization
Recently, we are also developing a method to find peptides with high activity by using reversible covalent bond formation reactions. Stabilizing a functional conformation can produce peptides with strong activity, but it is difficult to predict which chemical modifications are appropriate, and it is necessary to synthesize and evaluate a large number of candidate compounds. We are working to develop new methods that can streamline this process.
References:
• Y. Imamura, N. Umezawa, S. Osawa, N. Shimada, T. Higo, S. Yokoshima, T. Fukuyama, T. Iwatsubo, N. Kato, T. Tomita, T. Higuchi, "Effect of helical conformation and side-chain structure on g-secretase inhibition by b-peptide foldamers: Insight into substrate recognition."
J. Med. Chem., 56, 1443-1454, 2013.
• Y. Imamura, N. Watanabe, N. Umezawa, T. Iwatsubo, N. Kato, T. Tomita, T. Higuchi, "Inhibition of g-secretase activity by helical b-peptide foldamers." J. Am. Chem. Soc., 131, 7353-7359, 2009.
3 Design and synthesis of functional polyamine derivatives.
References:
• N. Umezawa, K. Tsuji, S. Sato, M. Kikuchi, H. Watanabe, Y. Horai, M. Yamaguchi, Y. Hisamatsu, T. Umehara, T. Higuchi, "Inhibition of FAD-dependent lysine-specific demethylases by chiral polyamine analogues." RSC Adv., 8, 36895-36902, 2018.
• M. A. Gulshan, K. Tsuji, S. Matsumura, T. Higuchi, N. Umezawa, Y. Ikawa, "Distinct modulation of group I ribozyme activity among stereoisomers of a synthetic pentamine with structural constraints." Biochem. Biophys. Res. Commun., 504, 698-703, 2018.
• T. Nishio, Y. Yoshikawa, W. Fukuda, N. Umezawa, T. Higuchi, S. Fujiwara, T. Imanaka, K. Yoshikawa, "Branched-chain polyamine found in hyperthermophiles induces unique temperature-dependent structural changes in genome-size DNA." ChemPhysChem, 19, 1-7, 2018.
• A. Muramatsu, Y. Shimizu, Y. Yoshikawa, W. Fukuda, N. Umezawa, Y. Horai, T. Higuchi, S. Fujiwara, T. Imanaka, K. Yoshikawa, "Naturally occurring branched-chain polyamines induce a crosslinked meshwork structure in a giant DNA." J. Chem. Phys., 145, 235103, 2016.
• N. Umezawa, Y. Horai, Y. Imamura, M. Kawakubo, M. Nakahira, N. Kato, A. Muramatsu, Y. Yoshikawa, K. Yoshikawa, T. Higuchi, "Structurally diverse polyamines: Solid-phase synthesis and interaction with DNA." ChemBioChem, 16, 1811-1819, 2015.
• Y. Yoshikawa, N. Umezawa, Y. Imamura, T. Kanbe, N. Kato, K. Yoshikawa, T. Imanaka, T. Higuchi, "Effective chiral discrimination of tetravalent polyamines on single-DNA compaction. "
Angew. Chem. Int. Ed., 52, 3712-3716, 2013.
Examples of polyamines existing in vivo and synthesized by us with solid-phase synthesis.
4 Development of synthetic receptors for heme in water.
References:
• Y. Hisamatsu, K. Otani, H. Takase, N. Umezawa, T. Higuchi, "Fluorescence response and self-assembly of a tweezer-type synthetic receptor triggered by complexation with heme and its catabolites", Chem. Eur. J., 2021, 27, 6489–6499.
• Y. Hisamatsu, N. Umezawa, H. Yagi, K. Kato, T. Higuchi, "Design and synthesis of a 4-aminoquinoline-based molecular tweezer that recognizes protoporphyrin IX and iron(III) protoporphyrin IX and its application as a supramolecular photosensitizer", Chem. Sci., 2018, 9, 7455-7467.
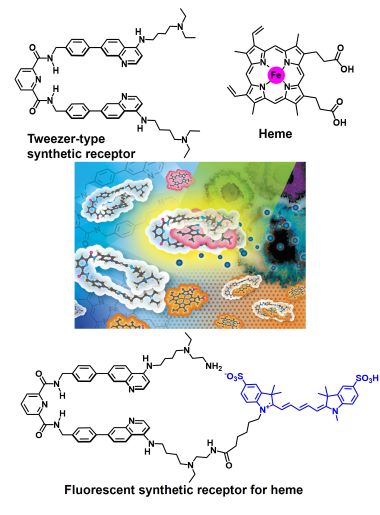
Synthetic receptors for heme
5 Development of amphiphilic 4-aminoquinoline-based building blocks and construction of diverse functional nanostructures in water.
References:
• Y. Hisamatsu, G. Toriyama, K. Yamamoto, H. Takase, T. Higuchi, N. Umezawa, "Temperature control of the self-assembly process of 4-aminoquinoline amphiphile: selective construction of perforated vesicles and nanofibers, and structural restoration capability", Chem. Eur. J., 2024. 30(36), e202400134.
• Y. Hisamatsu, F. Cheng, K. Yamamoto, H. Takase, N. Umezawa, T. Higuchi, "Control of the stepwise self-assembly process of a pH-responsive amphiphilic 4-aminoquinoline-tetraphenylethene conjugate", Nanoscale, 2023, 15, 3177-3187.
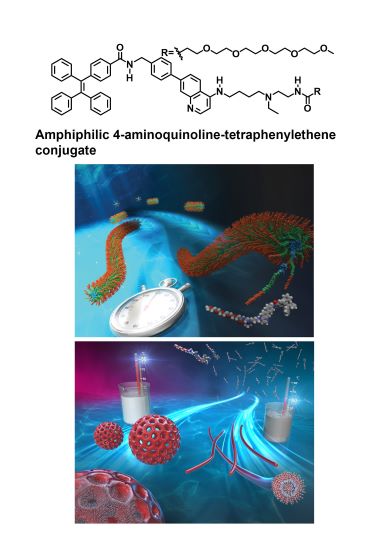
An example of an amphiphilic 4-aminoquinoline-based building block